May 24, 2017
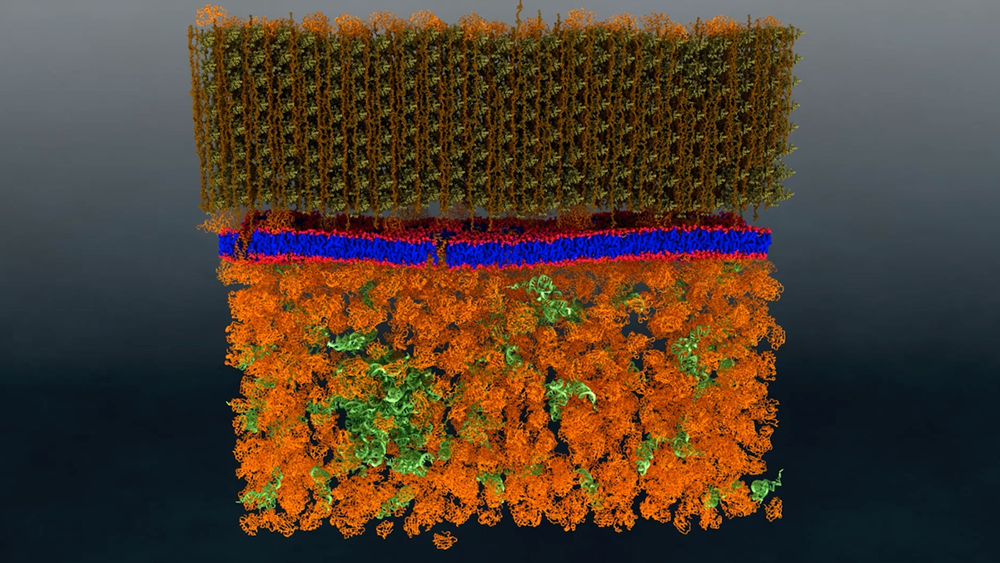
A research team from the Department of Energy’s Oak Ridge National Laboratory has performed the first-ever direct nanoscale examination of a living cell membrane. In doing so, it also resolved a long-standing debate by identifying tiny groupings of lipid molecules that are likely key to the cell’s functioning.
The methods developed provide a new experimental platform for biophysical studies of membranes and, potentially, other cell components. It could prove useful for future research on important interactions such as drug-membrane, biofuel-membrane, and even antibiotic-membrane interactions.
The multidisciplinary project—led by biophysicist John Katsaras, chemist Bob Standaert and microbiologist James Elkins—was performed at the lab’s High Flux Isotope Reactor and Spallation Neutron Source using the bacterium Bacillus subtilis. The team published its findings in the journal PLoS Biology.
A cell’s membrane is a thin bilayer of lipid molecules among which reside other biomolecules such as proteins. Researchers have been uncertain about whether membrane lipids sometimes organize into groups called domains, also known as “rafts,” or if they are randomly distributed in the membrane. Organization of lipids in distinct domains within the cell membrane is thought to enable functions such as signaling between cells.
“It became a debate,” Katsaras said. “Some people believed they exist, while others believed they didn’t. There was a lot of circumstantial evidence that could support either side.”
The problem was that existing techniques were not capable of unequivocally resolving this question.
Read more at ornl.gov/news.
Related Publication: Nickels, J.D., et.al. (2017). The In Vivo Structure of Biological Membranes and Evidence for Lipid Domains. PLoS Biology 15(5), e2002214. doi.org/10.1371/journal.pbio.2002214