April 8, 2022
Scientific Achievement
This study demonstrates a potential kinetic route to develop highly stable and selective MOFs for the adsorption of caustic acid gas species.
Significance and Impact
The work shows how the effect of spin-orbit coupling can hybridize dipolar and quadrupolar fluctuations, leading to unexpected quantum excitations despite the classical ground-state, a result that may be applicable to a range of other materials with hitherto unexplained multipolar excitations.
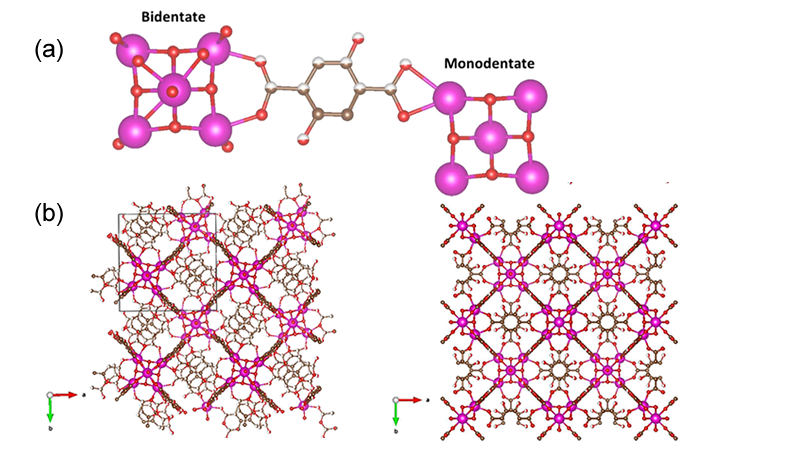
Research Details
- Optimized hydrothermal synthesis procedures developed to produce new family of MOFs.
- Single crystal and powder X-ray diffraction determined the crystal structure.
- High resolution neutron powder diffraction data was used to gain details of the ligand structure and disorder.
- Density-functional theory (DFT) calculations were used to investigate the binding energies of the acid gas molecules.
Related Publication: Henkelis, S.E. et. al. (2021). Kinetically Controlled Linker Binding in Rare Earth-2,5-Dihydroxyterepthalic Acid Metal−Organic Frameworks and Its Predicted Effects on Acid Gas Adsorption. ACS Applied Materials Interfaces, 13, 56337-56347. https://doi.org/10.1038/s41567-020-01110-1
