June 1, 2019
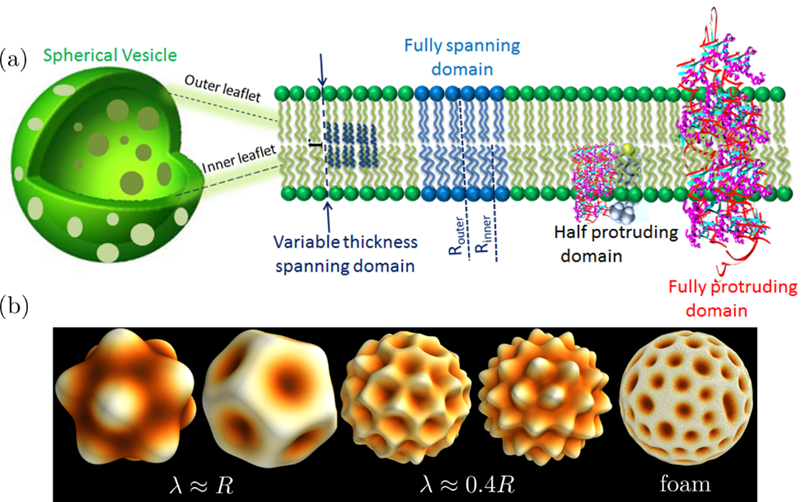
In biological membranes, functional domains or so-called lipid “rafts” have proven difficult to study systematically, owing in part to their chemical complexity and that of membrane composition, which often contains thousands of different biomolecules, including hundreds of distinct lipid species. Neutron scattering techniques have recently proven capable of studying nanoscopic lipid heterogeneities populating spherical vesicles. However, the development of analytical approaches capable of predicting and analyzing nanoscopic lipid phase separation from such experiments has not kept up with experiment. Importantly, neutron scattering techniques are among the few capable of providing nanoscale structural and dynamical information without the need of extrinsic probes (e.g., fluorescent labels) that are sometimes known to alter the interactions responsible for lipid rafts formation.
The coexistence of ordered and disordered fluid phases, their functionalities, and physiological significance in the presence of transmembrane proteins, peptides and hormones, to name a few, have yet to be fully addressed. Moreover, another fundamental question is how the two lipid bilayer leaflets interact and influence each other’s structure and dynamics under physiological conditions. Finally, to study the advent of lipid lateral organization we will combine new theoretical and computational frameworks, and state-of-the-art neutron scattering protocols.
Collaborations
ORNL; University of Tennessee, Knoxville; University of Pennsylvania; University of Windsor (Canada); University of Connecticut; Virginia Tech; University of Cincinnati; University of Delaware; University of Graz (Austria); University of Waterloo (Canada); Brookhaven National Laboratory; NIST; University of Texas Health Science Center at Houston; Ohio State University