September 4, 2020
SARS-CoV-2, the coronavirus responsible for the disease COVID-19, is infecting the world at a rapid rate. Understanding how this infection works at the molecular level could help experts discover ways to moderate or stop the spread.
A team of scientists at the Department of Energy’s (DOE’s) Oak Ridge National Laboratory (ORNL) is using neutron reflectometry to do just that.
Neutrons are capable of probing biological materials under physiological conditions without damaging them. By harnessing these properties, the researchers can measure the infection dynamics of the virus as it happens.
Their mission is to get a detailed look at some of the first stages of infection occurring at the cell membrane. These findings will help the team test antiviral drug candidates that could disrupt this process. The data obtained from these experiments could also inform other studies focused on developing therapeutics and vaccines.
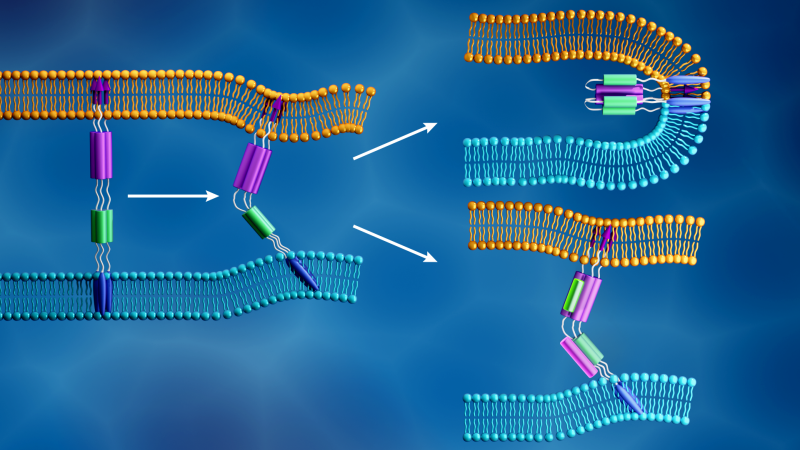
The researchers are focusing their analysis on SARS-CoV-2 spike proteins, barb-like structural proteins that cover the surface of the virus and trigger the infection process. The spike protein binds to a receptor on the host cell’s outer layer and facilitates fusion between the viral and cellular membranes, allowing the virus to enter the cell and release its genetic material. The cell’s protein-making machinery then uses this genetic information to make new copies of the virus.
When SARS-CoV-2 hijacks a host cell, its spike protein splits into two subunits, called S1 and S2. The two parts are both essential for infection. The S1 subunit contains a receptor binding domain that recognizes and latches on to a cell receptor. Cell receptors are proteins embedded within the cell membrane that can bind to specific molecules outside the cell. This connection can cause the components to change shape, which in turn could induce cascading changes within the cell. For the SARS-CoV-2 spike protein, this connection activates the S2 subunit, which helps the virus merge its membrane with that of the cell. Therefore, the spike protein’s function is similar to opening a locked door, with S1 as the key that unlocks the door and S2 as the force that pushes the door open.
Read more at neutrons.ornl.gov.