June 1, 2019
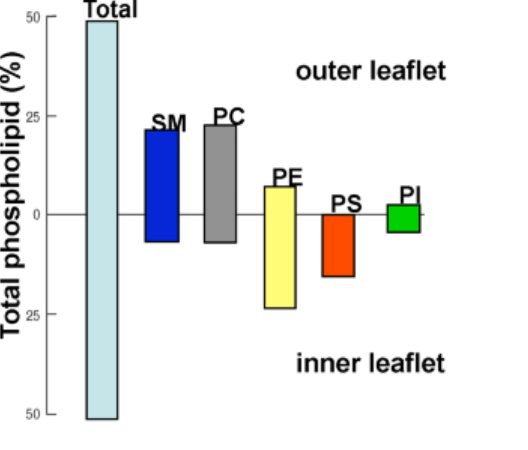
Transbilayer asymmetry is a ubiquitous yet poorly understood aspect of biological membranes. Increasing evidence suggests that the lipid compositional asymmetry of plasma membranes (PMs) not only confines specific lipids to the exoplasmic leaflet (in the case of sphingomyelin) or cytoplasmic leaflet (in the case of aminophospholipids), but also results in unique membrane properties that are almost certainly crucial for normal cellular function (Figure 1). The vast majority of biophysical studies make use of symmetric model membranes to study biomembrane characteristics, including structure and dynamics as well as the behavior of membrane proteins – even though these highly simplified models most likely fail to capture crucial aspects of the PM environment. With the use of a host of techniques, including neutron scattering, we aim to understand the structural and dynamic impact lipid asymmetry has on the PM.
Neutrons are an ideal tool for studying the structure and dynamics of asymmetric membranes. Selective lipid and solvent deuteration enables manipulation of interleaflet contrast such that SANS can “see” each leaflet separately, thus allowing for the independent structural determination of each bilayer leaflet. Importantly, deuterium (2H, a stable isotope of hydrogen) is practically identical to protium (1H) in its chemistry and replacing protium with deuterium has little effect on the system’s properties or behavior, making neutrons essentially a probe-free technique.
Challenges We Are Working On
Despite intense interest in asymmetric model membranes, many fundamental questions remain unanswered, including the basic question of how the two bilayer leaflets communicate with and influence each other’s structural and dynamical properties, or so-called interleaflet coupling. A related open question is the extent to which membrane asymmetry influences the function of transmembrane proteins – integral components contributing to the membrane’s functionality.
Collaborations
ORNL; University of Tennessee, Knoxville; University of Windsor (Canada); University of Graz (Austria); University of Texas Health Science Center; Cornell University; Weill Cornell Medical College; Stony Brook University